All disinfectants must undergo rigorous testing before commercial use, as determined by various regulatory bodies around the world.
The European Committee for Standardization (CEN), is a private and international non-profit organization responsible for standards creation. European standards are driven by business and made through a transparent, balanced, and consensus-based process in which relevant stakeholders are involved. CEN aims to produce high-quality standards for products and services that incorporate quality, safety, environmental, interoperability and accessibility requirements.
CENs Technical Committee 216 (TC216) was conceived in the late 1980s with a big scope: standardization of the terminology, requirements, test methods including potential efficacy under in-use conditions, recommendations for use, and labeling in the whole field of chemical disinfection and antiseptics. It is TC216, that works and publishes the most important document when it comes to disinfectants: the EN 14885, a bible of every Infection Prevention and Control Practitioner.
Whether you are an experienced IPC professional or you have just made your first steps in the field, the EN 14885 with its 82 pages of detailed procedures and rules can be overwhelming. However, standards and procedures are around us from the simple A4 format of the sheet of paper to complicated procedures of testing the microbial efficacy of disinfectants and biocides. So if you are still afraid of looking at never-ending tables or tired of scrolling through numeric names of the norms, we have done it for you and we are glad to present to you this vade-mecum.
This tool should help you in your daily decision-making or when you face difficulties to compare one product to another.
To feel comfortable with this document, there are a few things you should know beforehand :
FIELDS
EN 14885 describes 3 fields and the standard that correspond to them. Besides the medical area which this document covers, there are a bunch of norms that correspond to the veterinary area or food, industrial, domestic and institutional areas. If you don’t find a specific norm in this document probably the norm you see is not specific to the medical area.
STEPS AND PHASES
Testing is divided into 3 phases. Phase 1 tests are quantitative suspension tests to establish that a product under development has bactericidal, fungicidal, yeasticidal, or sporicidal activity without regard to specific areas of application. Phase 1 tests cannot be used for any product claim. Phase 2 comprises two steps:
a) Phase 2, step 1 tests are quantitative suspension tests to establish that a product has specific activity under simulated practical conditions appropriate to its intended use (in vitro);
b) Phase 2, step 2 tests are quantitative laboratory tests to establish that a product has specific activity when applied to a surface or skin under simulated practical conditions (e.g. surface, instrument, laundry, handwash, and hand rub tests);
Phase 3 tests are field tests under practical conditions. Applicable methodologies for this type of test are not yet available but may be developed in the future.
WHAT IS ESSENTIAL AND WHAT ISN’T
All products in the medical area shall pass the standards for bactericidal and yeasticidal activity as a minimum requirement. Remaining efficacies can be used for additional safety, marketing, or competitive advantage or when the facility needs them (problems with reoccurring outbreaks, or specific pathogen presence).
TERMINOLOGY
The name of the norm corresponds to EN (European Norm) and the number followed by the year of publishing or a total revision (for example EN 17272 2020). Sometimes, you will find further +A1 with another date or +A2. This will mean that before a total revision of the norm (usually every 4 years) an amendment (small correction or revision) was executed on the norm.
ORGANISMS
Test organisms are chosen to mimic the most relevant organisms for each activity. This is a tricky task, as we also should use the organisms well studied, with known mechanisms of resistance to biocides or antibiotics, so we can predict possible mutation from non-virulent to virulent variants. Concerning viruses, for the safety of people performing the study the strains used should be of low virulence to avoid possible infection of the personnel.
SOILING
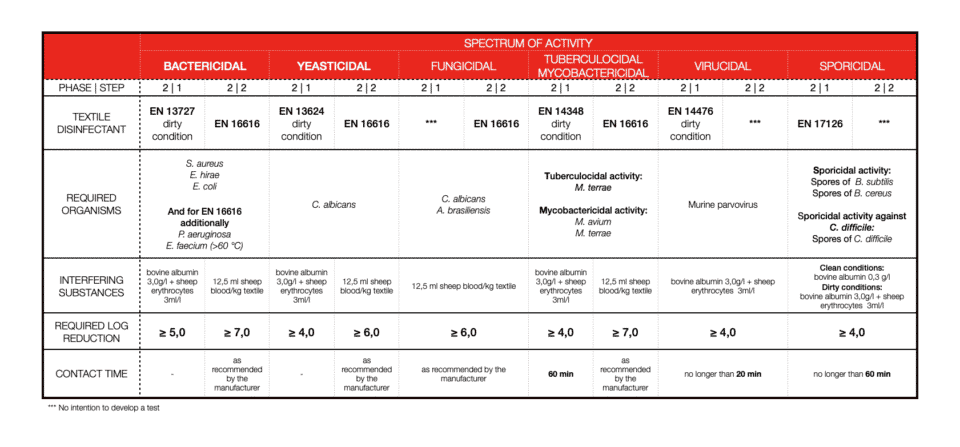
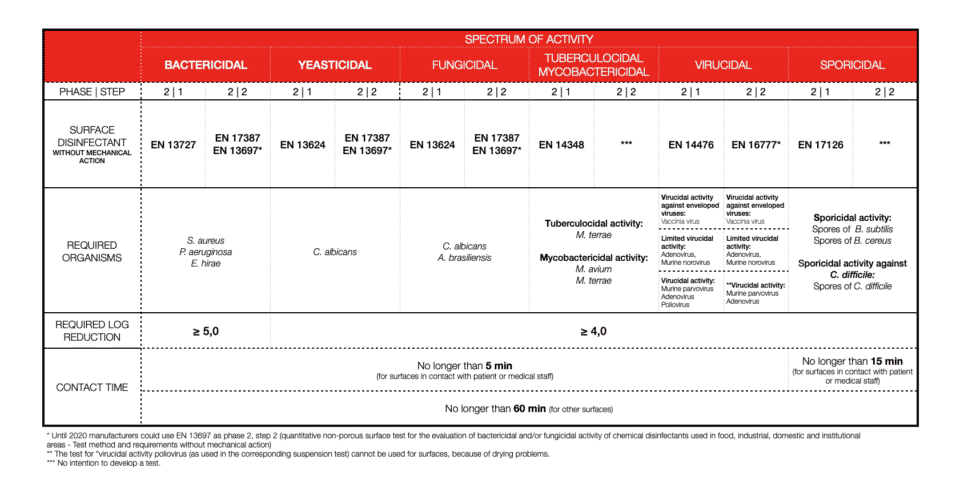
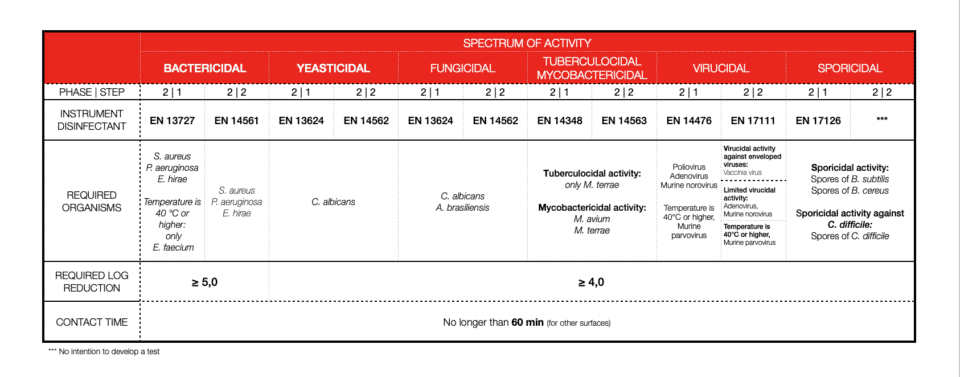
Soiling or so-called interfering substances are important. We can’t disinfect an item that is not clean, therefore 2-in-1 products (cleaners and disinfectants) should be tested in dirty conditions (always!). What we call clean condition is: bovine albumin: 0,3 g/l and dirty conditions concerns: bovine albumin: 3,0 g/l plus sheep erythrocytes: 3 ml/l. For textiles, disinfection soiling differs.
LOG REDUCTION
Bacterial log reduction is determined for a minimum of 5logs (99.999%) of bacteria killed. For other efficiencies, most of the time 4log reduction (99.99%) is required. Again, this is different for textile disinfection.
CONTACT TIME
Contact time with disinfectant is one of the most important factors. Short contact time means high activity. Depending on the field of application or product claim contact time will vary a lot. Also, the type of chemistry and its mode of action will not be the same for every microorganism. Now you are ready to explore the tables. As of 2022, all you need to know and pay attention to should be there.
EN 14885: VADE-MECUM FOR IPC PRACTITIONERS
HAND DISINFECTION

SURFACE DISINFECTION WITH MECHANICAL ACTION
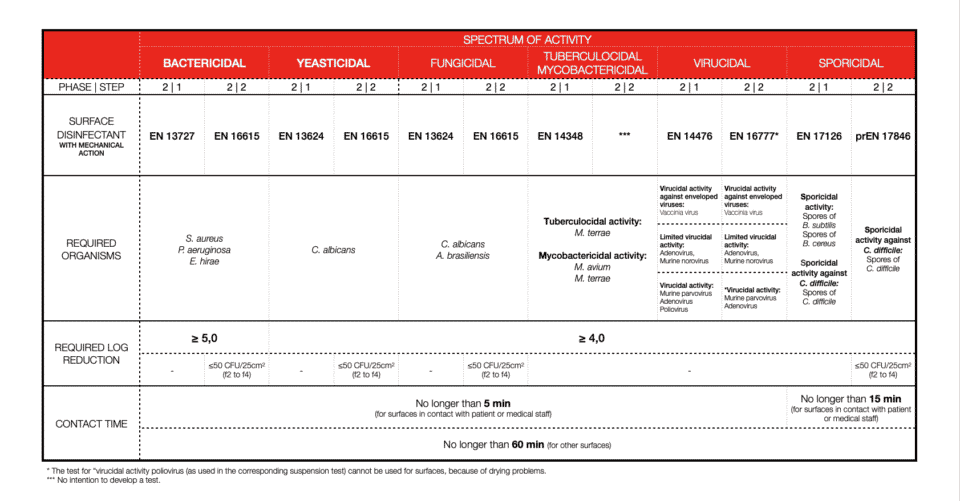
SURFACE DISINFECTION WITHOUT MECHANICAL ACTION
INSTRUMENT DISINFECTION
TEXTILE DISINFECTION